(Xinhua, Guozhong) On December 7, the journalNaturepublished the latest researchresultsof Yan Huan'sresearchgroup in the field of viral receptors intheNationalKey Laboratory of Virology, School of Life Sciences in the form of anarticle entitled"Close relatives of MERS-CoVin bats use ACE2 as their functional receptors" (《MERS冠状病毒在蝙蝠中的近亲病毒使用ACE2作为它们的功能性受体》).

Researcher Yan Huan oftheNationalKey Laboratory of Virology, School of Life Sciences, and Taikang Center of Life Sciences, Wuhan University, and Researcher Wang Xiangxi oftheInstitute of Biophysics, Chinese Academy of Sciences, and Professor David Veesler oftheDepartment of Biochemistry, University of Washington, USA are the co-corresponding authors of the paper.PhD student Xiong Qing andmaster’sstudent Ma Chengbao oftheCollege of Life Sciences, Wuhan University, Associate Researcher Cao Lei oftheInstitute of Biophysics, Chinese Academy of Sciences, and Dr. M. Alejandra Tortorici, Department of Biochemistry, University of Washington, USA, are the co-first authors of the paper.

Photo of Yan Huan's research group (Yan Huan is the 6th from the right)
NeoCoV was discovered in Cape serotine samples from South Africa in 2012 and is the closest coronavirus to MERS-CoV that has been identified in nature. In exploring the functional receptors of multiple representative bat coronaviruses, Huan Yan's group found that NeoCoV and PDF-2180, two pseudoviruses of MERS-associated coronaviruses, were able to enter cellsexogenouslyexpressing the human ACE2 receptorwith low efficiency.It is already well known in the field that ACE2 (angiotensin-converting enzyme 2) is the receptor for coronaviruses of the Sarbecovirus subgenus,such as NeoCoV SARS-CoV-2. This unexpected result attractedconsiderableattention of the research group. Using a pseudovirus system, the researchers further tested 46 bat ACE2 receptors and found that both viruses could more efficiently bind the ACE2 receptors of a variety of positive pteropod bats into cells. This result breaks through the current conventional knowledge of coronavirus receptors and reveals for the first time that MERS-associated coronaviruses can enter cells using ACE2 rather than DPP4 (dipeptidyl peptidase 4, the receptor for coronaviruses of the subgenus Merbecovirus such as MERS-CoV).

Kinship, natural host and receptorusageof some representative coronaviruses
On this basis, Wang Xiangxi's team at the Institute of Biophysics, Chinese Academy of Sciences has successfully resolved the high-resolution structure of the ACE2 complex with the RBD of the two viral stinging proteins of Pipistrelluspipistrellusby cryo-electron microscopy, and David Veesler's team at the University of Washington has also resolved the high-resolution structure of the PDF-2180 stinging protein full-length trimer by cryo-electron microscopy. The high-resolution structure of the full-length trimer of PDF-2180 spinosin was also resolved by cryo-electron microscopy by David Veesler's team at the University of Washington. Structural analysis revealed that, unlike SARS-CoV, SARS-CoV-2 and NL63, which also use ACE2 receptors, NeoCoV and PDF-2180 recognize ACE2 using a novel binding mode that relies on protein-glycosyl interactions. The footprint of NeoCoV and PDF-2180 was also significantly different compared to the other three viruses, suggesting that the more distantly related coronaviruses had convergently evolved to independently select and adapt to ACE2 as a receptor during their evolutionary history.
Subsequently, the researchers further collaborated to explore the molecular mechanisms of receptor recognition by these two viruses and the ability of the associated antibodies to block pseudovirus entry into cells. Sequence analysis and mutation studies revealed that the sequence difference in amino acids 338-342 on the RBD binding blot of human ACE2 relative to that of ambulant bat ACE2 was the key reason for its poor ability to mediate infection, but NeoCoV could achieve efficient recognition of human ACE2 by a single point mutation in T510F on its stinging protein. Antibody neutralization experiments showed that neither serum from NeoCoV vaccine recipients nor monoclonal antibodies targeting MERS-CoVRBD could inhibit NeoCoV and PDF-2180 pseudovirus infection under the current experimental conditions, whereas a specific antibody targeting ACE2 (H11B11) and two broad-spectrum neutralizing antibodies against β genus coronaviruses (B6 and S2P6) could effectively block the entry of pseudoviruses of these two viruses into the cells.
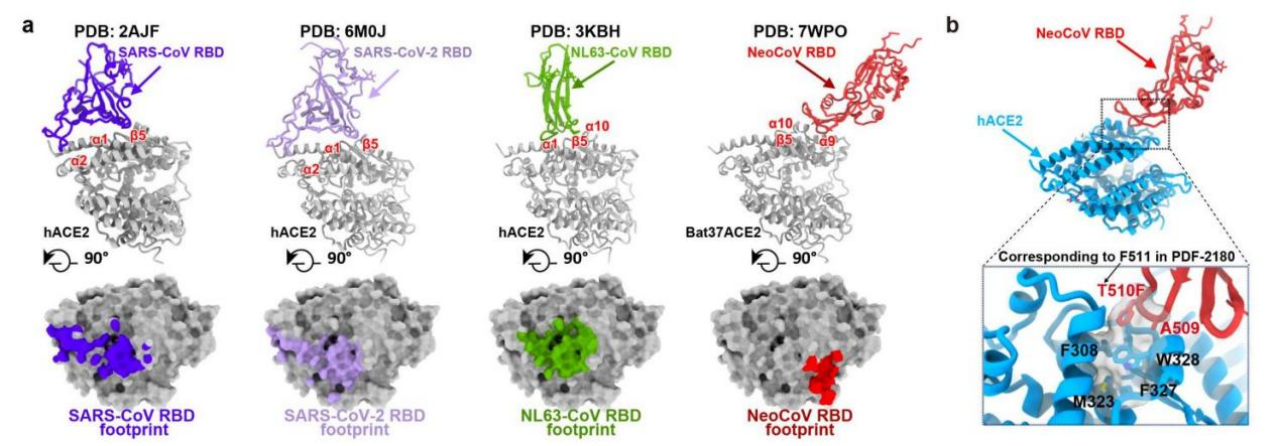
Interaction of different coronavirus RBDs and their patterns of binding ACE2 (a) and NeoCoVRBD carrying T510F mutation with human ACE2 (b)
In summary, this study reveals that the functional receptor for two bat MERS-associated coronaviruses (NeoCoV and PDF-2180) is ACE2, and resolves the structure of the receptor-binding complexes of these two viruses as well as the PDF-2180 stinger protein trimer, suggesting a potential threat to human health from this class of viruses and providing new evidence for the bat origin hypothesis of MERS-CoV. The results of this study will help to further promote the use of ACEs to targethuman health risk and the basic research on MERS-associated coronaviruses using the ACE2 receptor, as well as lay the foundation for the development of related vaccines and antiviral drugs. Finally, it should be noted that all infection experiments in this study involved low-risk pseudoviruses and did not involve real coronaviruses or genetic modification of real viruses.
This study was funded by the National Natural Science Foundation of China, the Wuhan University New Coronavirus Research Special Fund, the Strategic Key Research Program of the Chinese Academy of Sciences, and the National Key Research and Development Program.
Rewritten by Xuying Cai
Edited byKeke Liang, Sylvia, Xi Bingqing
Link to paper:https://www.nature.com/articles/s41586-022-05513-3